- Home >
- Science
- > Technology
How Does Soap Work?
Soap works by breaking down and removing dirt and grease from surfaces. It is made up of molecules with hydrophilic (water-attracting) heads and hydrophobic (water-repelling) tails. When you apply soap with water, the hydrophobic tails attach to the oils and fats, while the hydrophilic heads remain in the water. This interaction creates micelles, trapping the dirt and allowing it to be rinsed away, leaving surfaces clean.
Advertisement

Understanding How Soap Works: A Scientific Perspective
Soap is an essential part of our daily hygiene routine, yet few of us truly understand how it works. Soap, a humble household product, plays a crucial role in maintaining our health by effectively cleaning our skin and various surfaces. The process behind its ability to cleanse and disinfect is both fascinating and scientifically grounded. By exploring the chemistry of soap, we can better appreciate its importance in our lives.
Soap is a type of surfactant, meaning it has the ability to reduce the surface tension of water, making it easier to spread and interact with other substances. This is due to the unique structure of soap molecules, which have two distinct ends: a hydrophilic (water-attracting) head and a hydrophobic (water-repelling) tail. When soap is mixed with water, the hydrophobic tails avoid the water and attach themselves to oils and grease, while the hydrophilic heads remain in the water.
This dual-action allows soap to effectively break down and lift away dirt, oils, and microbes from surfaces. The soap molecules form structures called micelles, which encapsulate the dirt and oils, suspending them in water so they can be easily rinsed away. This process not only removes visible grime but also helps eliminate many bacteria and viruses, making soap an effective tool in reducing the spread of infectious diseases.
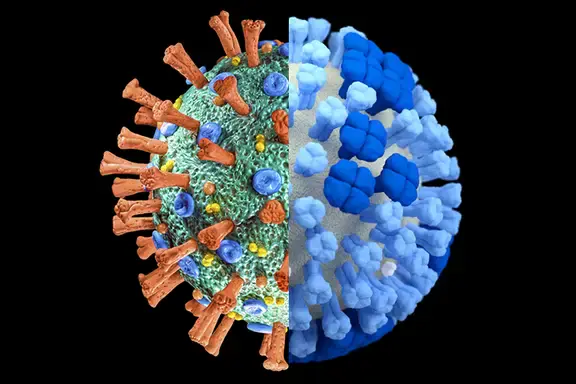
The effectiveness of soap is further enhanced by its ability to disrupt the lipid membranes of certain pathogens, such as viruses and bacteria. Many viruses, including the coronavirus, have an outer lipid layer that is essential for their survival. The hydrophobic tails of soap molecules can penetrate this lipid layer, effectively breaking it apart and rendering the virus inactive. This is why handwashing with soap and water is highly recommended by health authorities as a primary defense against infectious diseases.
In addition to its cleansing properties, soap also contributes to skin health. Unlike harsh detergents, soap is typically milder and less likely to strip the skin of its natural oils. This makes it suitable for regular use without causing excessive dryness or irritation. Moreover, many soaps are formulated with added ingredients, such as moisturizers and essential oils, to further nourish and protect the skin.
While soap is a powerful cleaning agent, it is important to use it correctly to maximize its effectiveness. Proper handwashing techniques, which include scrubbing all parts of the hands for at least 20 seconds, ensure that all dirt and microbes are effectively removed. It's also crucial to rinse thoroughly with clean water to wash away the micelles and the trapped particles they contain.
In conclusion, soap is a remarkable product that combines simple chemistry with practical application. Its ability to clean, disinfect, and maintain skin health makes it an indispensable part of everyday life. By understanding how soap works, we can better appreciate its role in promoting health and hygiene, reinforcing its importance in our daily routines.