- Home >
- Science
- > Innovation
Why Does Salt Melt Ice?
Salt melts ice by lowering its freezing point, a process known as freezing point depression. When salt is added to ice, it dissolves into the thin layer of water on the ice's surface, creating a saltwater solution. This solution disrupts the equilibrium between the ice and water, preventing the water molecules from forming solid ice at the usual freezing temperature. As a result, the ice melts, even if the temperature remains below the normal freezing point of water.
Advertisement
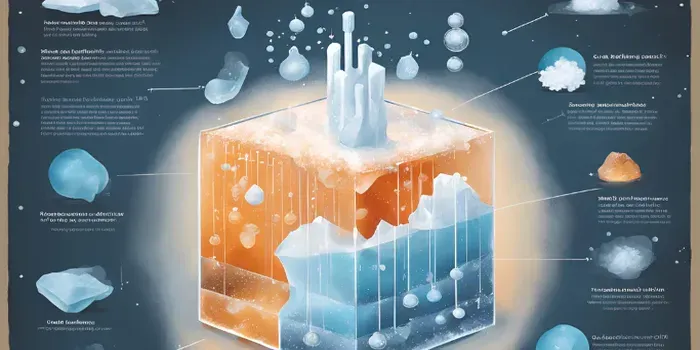
Understanding the Science Behind Salt's Ice-Melting Power
When temperatures drop and roads become treacherous with ice, many municipalities and homeowners turn to salt as a reliable solution for melting ice. But how exactly does this everyday kitchen staple work its magic on frozen surfaces? The answer lies in a fascinating interplay of chemistry and physics, which not only highlights the effectiveness of salt but also underscores its importance in winter road maintenance.
At its core, the process by which salt melts ice is a simple yet intriguing chemical reaction. Ice is water in its solid state, and its molecules are bound together in a rigid structure. For ice to transition back into liquid water, these bonds need to be disrupted. This is where salt comes into play. When salt is sprinkled over ice, it dissolves in the thin layer of liquid water that is always present on the surface, even at temperatures below freezing. This action effectively lowers the freezing point of the water, a phenomenon known as freezing point depression.
The science of freezing point depression is critical to understanding why salt is so effective. Normally, water freezes at 32 degrees Fahrenheit (0 degrees Celsius). However, when salt is added, it interferes with the ability of the water molecules to form solid ice. As a result, the freezing point of the water-salt mixture is lowered, sometimes significantly. This means that even if the ambient temperature is below the normal freezing point of water, the water will remain in a liquid state, allowing more ice to melt.
Different types of salt can be used to melt ice, with sodium chloride (table salt) being the most common. Other salts like calcium chloride and magnesium chloride are also effective and can work at even lower temperatures than sodium chloride. Each type of salt has its unique properties and effectiveness, making them suitable for various environmental conditions.
Beyond the chemistry, the practical use of salt for melting ice is widespread due to its cost-effectiveness and efficiency. It helps to prevent accidents by reducing ice formation on roads and walkways, thereby ensuring safer conditions for vehicles and pedestrians. However, it's worth noting that while effective, excessive use of salt can have environmental impacts, such as soil degradation and harm to aquatic life. Therefore, it's crucial to use salt responsibly and explore alternative ice-melting solutions when possible.
In conclusion, the ability of salt to melt ice is a testament to the fascinating principles of chemistry and physics. By lowering the freezing point of water, salt effectively transforms icy surfaces into safer passageways during the harsh winter months. As we continue to rely on this method for road safety, understanding the science behind it allows for more informed and environmentally conscious usage.